3D printed organs could soon become a reality
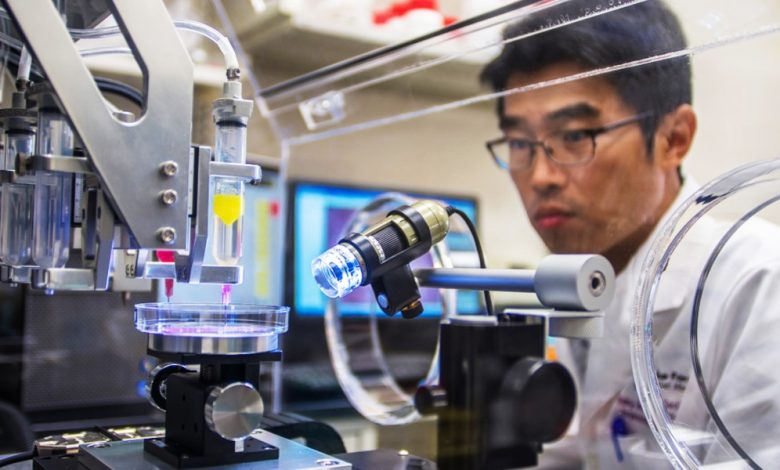
Last year, Dr. Arturo Bonilla in San Antonio, Texas, carefully an outer ear to a 20-year-old woman who was born without one. The ear on the woman’s right side was engineered to be the size and shape of her left.
For Bonilla, a pediatric microtia surgeon (a doctor who treats birth defects of the ear) for more than 25 years and a recognized expert in the field, such a procedure would normally be routine. But this case had a twist: For the first time, the ear he implanted was the product of a 3D bioprinter using the woman’s own cartilage cells.
The implantation procedure, Bonilla told me, was “very uneventful.” It’s a huge understatement, all things considered.
From the realm of near-fiction to the germ of an idea to actual science, 3D bioprinting is advancing in every aspect of medical research – and now practice. The pace is slow and target dates for some of the most ambitious 3D plans are decades away. But progress is real.
“I think that in ten years we will have organs for transplantation,” says Professor Tal Dvir, director of tissue engineering and regenerative medicine at Tel Aviv University in Israel. “We will start with simple organs like skin and cartilage, but then move on to more complicated tissues – eventually heart, liver, kidney.”
The future of 3D bioprinting
It sounds fantastic, but it’s already happening. Layered skin, bones, muscle structures, blood vessels, retinal tissue, and even mini-organs have all been 3D printed. While none of the printed products have yet been approved for human use, the race down the scientific timeline is breathtaking – and Bonilla’s ear procedure, the first 3D bioprint of living cells to be implanted in a human, marks a significant moment in that progress.
Researchers in Poland have bioprinted a functional prototype pancreas in which stable blood flow was achieved in pigs over an observed period of two weeks, according to a 2022 abstract and Dr. Michal Wszola, creator of Bionic Pancreas. United Therapeutics Corporation has 3D printed a human lung scaffold in animal models with 4,000 kilometers of capillaries and 200 million alveoli (tiny air sacs) capable of oxygen exchange – a critical step in creating compatible, transplantable human lungs with the goal of being cleaned for human trials within five years.
Scientists at the Wake Forest University Institute for Regenerative Medicine have developed a mobile skin bioprinting system. They envision that in the not-too-distant future, they will be able to wheel the printer right to the bedside of a patient suffering from a non-healing wound such as a burn, then scan and measure the wound area, and 3D print layers of skin layer-by-layer right on the wound surface. And they’ve gone deeper by creating 3D printed skeletal muscle constructs that have been shown to contract in rodents and regain more than 80% of previously lost muscle function in a front leg muscle within eight weeks.
Dvir’s own lab has produced a 3D-printed “rabbit-sized” heart, as he puts it, crammed with cells, chambers, major blood vessels, and a heartbeat. Full-size human hearts, the professor says, require the same basic technology, although the process of scaling is extremely complicated. “We are now working on the pacemaker cells, the atrial cells, the ventricular cells,” says Dvir. “But it looks good. I think that’s the future.”
How 3D bioprinting works
Courtesy of WFIRM
The ability to 3D print human organs is an amazing concept. Nearly 106,000 Americans are currently on organ donation waitlists, and 17 die every day while waiting, according to the Federal Health Resources and Services Administration. A 3D printing process that uses the patient’s own cells to grow organs would not only potentially curb that waiting list, but also drastically reduce the likelihood of organ rejection and likely eliminate the need for harmful lifelong immunosuppressive drugs.
“The ability to place different cell types in precise locations to build a complex tissue and the ability to integrate blood vessels that can deliver the necessary oxygen and nutrients to keep the cells alive are two (3D) techniques , revolutionizing tissue engineering. says Mark Skylar-Scott, an assistant professor in Stanford University’s Department of Bioengineering. “The field has evolved very rapidly over the past two decades, from printed bladders to now highly cellular tissues with vessels that can be connected to a pump – and complex 3D models resembling heart components with integrated heart cells.”
In 3D bioprinting, the game is called cells. The process begins by generating the cells the researchers want to bioprint, which are then instructed to become organ-specific cell types. The cells are then turned into a printable living ink, or bioink, where they are mixed with materials like gelatin or alginate to give them a toothpaste-like consistency. Stanford’s lab is studying how stem cells might naturally form such a consistency when crammed together at high density, which could result in 3D-printed organs made entirely of a patient’s own cells.
The bio-ink is loaded into syringes and squeezed out of a nozzle “like icing on a cake,” says Skylar-Scott. This is the actual 3D bioprinting process and typically involves laying down different cell types, each loaded into a different nozzle. (Dvir says the mini-heart took about four hours to print.) Once it’s done, the tissue is sometimes attached to a pump that pushes oxygen and nutrients through the heart. Over time, the tissue develops on its own, increasing in both maturity and function.
This general process, although greatly simplified here, led to the fabrication of the outer portion of the ear that Arturo Bonilla implanted in his Texas patient. In most previous microtia surgeries, Bonilla would have carved a new ear out of cartilage harvested from the patient’s ribs. Instead, a small biopsy was taken from the patient’s other ear, and cartilage cells taken from the biopsy were grown into billions of cells that were 3D printed into the new implant.
“As with any study, there will likely be iterations in future patients to try to improve this technique,” says Bonilla. “We’re not sure when this will be the main treatment, but the future is very exciting.”
Benefits of 3D printing
Courtesy of WFIRM
Wake Forest scientists have been growing organs and tissues in the lab for years. They used 3D printing to essentially create a mini-kidney and mini-liver in the lab. The next challenge: larger, solid structures that better mimic organ function. “We’re a long way from reaching that goal at the organ level,” says Jennifer Lewis, Wyss Professor of Biologically Inspired Engineering at Harvard University.
“We could print flat structures like skin, tubular structures like blood vessels, or hollow non-tubular organs like a bladder,” says Anthony Atala, founding director of the Wake Forest Institute. The larger solid organs are different, Atala says, “because of the challenge with vascularity or nutrition. There are so many cells per centimeter.”
In a way, cell production is a quality issue. Scientists have managed to create a heart cell from stem cells, but not one that beats as hard as your heart cells. The same applies to liver cells (metabolism) and kidney cells (filtrate uptake). “In a way,” says Skyler-Scott, “the field of 3D bioprinting is waiting for basic biologists to make their big breakthroughs.”
There is also the problem of crowds. Making a heart would require “billions of cells — and you need different cells, even different heart cells,” says Tal Dvir. According to Skyler-Scott, to make enough cells for a single organ, a facility would need a 10-liter stirred tank, which could cost $5,000 a day to feed for months. And the ultimate goal is thousands of organs a month, not one.
It also raises questions about how tissue integrates into and is supported by the body, including complex networks of blood vessels, nerves and multiple cell types, says Dan Cohen, CEO and co-founder of 3D Bio Therapeutics. “That’s not to say it can’t be done,” says Cohen, who started working in the field of bioprinting 20 years ago, before it had a formal name. “I have a lot of hope for bioprinting and regenerative medicine more broadly.”
Significant progress has also been made in the short term. Researchers at Harvard, Lewis says, created heart cells from human pluripotent stem cells and then put them on a bioengineered chip with built-in sensors that can track the beating tissue. This 3D printed heart on a chip can be used to test various heart drugs for potentially toxic side effects and may reduce the need for animal testing ALS-on-a-chip technology will be used to screen for drug candidates and better understand the underlying mechanisms of this disease.)
“The 3D printer gives you several advantages,” says Atala of Wake Forest. “The first is scaling, because instead of making these (tissues and organs) one by one by hand, you can automate the printer to do it. The second is precision. We can locate the cells more precisely where they are needed.”
There’s also the notion of a lower overall cost since 3D printing allows for this larger scale. There is what Atala calls “reproducibility,” a method of making the same structure over and over again. And in terms of organ transplants, a new organ made from a patient’s own cells makes rejection far less likely.
Most researchers put the idea of a full-size 3D-printed organ transplant in humans at between 20 and 30 years. “Looking ahead, at some point we will no longer need donor hearts. We don’t need livers,” says Dvir. “That’s my opinion, and I’m optimistic, but I think in less than 20 years we’ll have printed organs inside us.” This is science at work, not science fiction.



